STEELS
IRON-CARBON Phase Diagram or Equilibrium Diagram
We all know that a Pure Iron metal is very weak in terms of strength. So, different metals were added to pure iron to form new metal (alloy) & increase its strength.The term Phase means, the stages of material at any condition like Solid, Liquid, gaseous ( a Homogeneous portion of a system having a single composition & same physical & chemical properties throughout its volume).
Ex. solid Phase, Liquid Phase etc.,
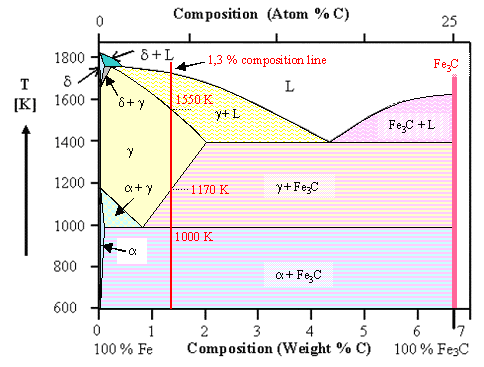
In Iron Carbon phase diagram, we learn mainly about the composition Iron & Carbon (Fe & C) to form STEEL. Steel is an alloy formed by adding 0.8% to 2% of carbon to Iron metal.
if the carbon composition is from 2% to 6.67% it is called as "Cast Iron".
From the above Phase diagram, we read all the properties of steel, during its formation by composition of Iron & Carbon.
We mainly study the changes of phases from solid to liquid with increasing in temperature vice versa.
In the above diagram, the phases were named as α, γ, δ and Fe3C with Iron corresponding to their temperatures & carbon Mixture .
1.
α = Ferrite
(the interstitial solid solution of carbon in α-iron)
2.
γ = Austenite
(the interstitial solid solution of carbon in γ-iron)
3.
δ = Perlite
(It is the mixture of Ferrite +Liquid metal)
Fe3C = Cementite
( Iron-carbon alloy in the form of chemical compound (Fe3C) Ferrite: It is the Mixture of carbon from 0.008% to 0.025% in α-iron (Temp. ranges from 0-723oC)
Austinite: It is the mixture of carbon in γ-iron (temp. ranges from 723° to 1493°). It consists upto 2% of Carbon. Austenite phase starts at 0.83% carbon at 723°C & completes its phase 1493°C at 0.16%carbon. At 1147°C with 2%Carbon Austinite Phase exhibits steel properties.
Cementite: It is defined as the mixture of iron with carbon of 6.67%. This composition of iron with carbon of 2% to 6.67% is called Cast Iron.
Perlite: It is the solidus solution of Ferrite & Cementite.
Ledeburite: It the solidus solution of Austenite & Cementite.
Eutectic Point: The interstitial (middle) point between Ferrite & Austinite at 723°C , where the steels having 0.83% carbon is called Eutectic Point.
Hypo Eutectic: The interstitial region b/w Ferrite & Austinite at 723°C, where steels havin less than 0.83%Carbon is called Hypo-Eutectic.
Hyper Eutectic: The interstitial region b/w Ferrite & Aistinite at 723°C, where steels having More than 0.83%Carbon is called Hyper-Eutectic.
Eutectoid: The interstitial point between Austinite & Liquidus state at 1147°C , where the steels having 4.3% carbon is called Eutectoid.