Heat:
It is
defined as the energy transferred, without transferring of mass across the
boundary of a system. This phenomena occurs because of temperature difference
between system and surroundings.
- It is denoted by Q and expressed in Joule (J)
Heat can be
transferred in 3 ways
- Conduction
- Convection
- Radiation
Conduction:
Transmission of heat through a Sold body is called conduction.
Convection: Transmission
of heat through Fluid is called convection.
Radiation: Transmission
of heat through Electromagnetic waves is called radiation.
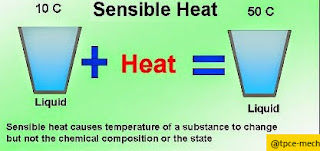
*Sensible
Heat: when a
body is heated its temperature rises.
The increase
in heat in a body is called Sensible Heat. Similarly when we remove heat from a
body, the fall or decrease in heat is also called as Sensible Heat.
*It is defined as the amount of heat is removed or added to a substance without change in its state.
Units: kJ/kg-k
Mathematically it is given as
*It is defined as the amount of heat is removed or added to a substance without change in its state.
Units: kJ/kg-k
Mathematically it is given as
Qs=mCp(ΔT)
where Qs= sensible heat
m= mass of substance
Cp= specific heat
ΔT= change in temperature
- *It is denoted by hf
*Latent Heat: The heat
which brings changes in states in a substance with no change in temperature is
known as Latent Heat.
Every pure
substance has the ability to change in its state from Solid to liquid to
gaseous state.
Mathematically it is given as
m= mass of substance
L= Latent heat of substance
Note: Latent heat of fusion of ice is 336kJ/kg
Latent heat of Condensation (Vapour-water) is 2258 kJ/kg
Mathematically it is given as
QL=mL
Units: kJ/kg-k
QL =Latent Heatm= mass of substance
L= Latent heat of substance
Note: Latent heat of fusion of ice is 336kJ/kg
Latent heat of Condensation (Vapour-water) is 2258 kJ/kg
- *It is denoted by hfg
Ex: Ice
changes to water, water changes to vapor(gas) at same temperature. ie.,100oC
*Specific Heat: It is
defined as the amount of heat requited to raise 1 degree of temperature to a
mass of a substance.
It is
denoted by C. Unit of specific heat is taken as KJ/kg
=>If m kg of a substance of specific
heat is required to raise temperature from initial temperature T1 to
final temperature T2, then heat required is
Q=mC(T2-T1)
kj
Specific heat of Water is 4.18 kJ/kg